Denali's Investigational Treatment

Denali is investigating an innovative, oral treatment called DNL151 that aims to slow disease progression and treat the underlying cause of the disease.
What is DNL151?
DNL151 is an investigational, oral treatment currently being studied as a treatment for the underlying causes of Parkinson’s.
DNL151 has been shown to cross the blood-brain barrier, delivering treatment to the brain.
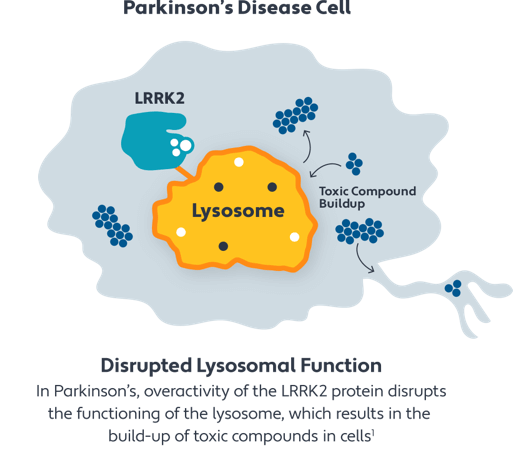
DNL151 is thought to work by blocking activity of the LRRK2 protein. Overactivity of the LRRK2 protein is responsible for disrupting the normal functioning of the lysosomes, the special compartments within cells that break down excess or worn-out cell parts. Disrupted lysosome functioning may lead to the build-up of toxic compounds that are commonly found in Parkinson's.
By blocking the activity of the LRRK2 protein, DNL151 has the potential to restore normal functioning of the lysosomes and therefore the removal of these toxic compounds, which may lead to slowing the progression of Parkinson's.
Denali will be enrolling people diagnosed with Parkinson's in a clinical trial on DNL151 in the near future. Register to receive updates on Denali Therapeutics' investigational treatments, research in Parkinson's, and future clinical trials.
References: 1. DiMaio R, et al, Sci Transl Med. 2018; 10:eear5429
DNL151 is an investigational drug and is not approved by any Health Authority